
订购NovaSeq X系列
将先进的化学、光学和信息学技术相结合,实现了超凡的速度和数据质量、出色的通量和可扩展性。
使用涵盖所有主要变异类别以及来自FFPE组织的基因特征(TMB、MSI和HRD)的大型泛癌panel实现CGP。
分析时间
手动操作时间
起始量
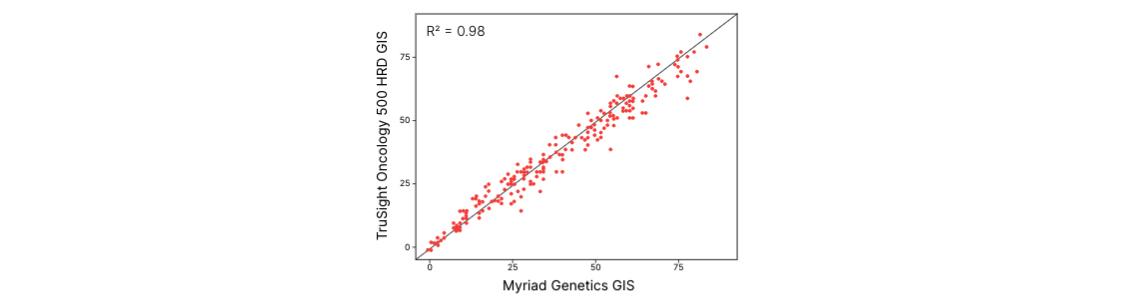
TruSight Oncology 500 HRD现已上市。通过可靠、准确的同源重组缺陷(HRD)评估实现全面的基因组分析。暂未在日本推出。
TruSight Oncology 500(TSO 500)是一种NGS检测方法,可从FFPE组织进行内部全景基因组分析(CGP)。
评估>500种与指南和临床试验一致的泛癌生物标志物,通过单次检测增加发现阳性生物标志物的机会。使用可选的附加HRD试剂盒评估基因组不稳定性。†
使用手动或自动化工作流程,通过本地服务器或在云端使用Illumina Connected Analytics与即用型DRAGEN二级分析流程集成,可在4-5天内获得结果。通过多种选项获取见解和生成报告,包括Illumina Connected Insights‡或Pierian。
*不适用于TSO 500 HRD。
†HRD仅适用于在TruSight Oncology 500中添加TruSight Oncology 500 HRD试剂盒的情况。暂未在日本推出。
‡仅在部分国家/地区上市。Illumina Connected Insights通过对第三方知识库的API调用,支持用户自定义的三级分析。
使用TruSight Oncology 500产品系列鉴定用于临床研究的疾病相关生物标志物。
TruSight Oncology 500
通过辅助试剂盒评估FFPE肿瘤组织的相关DNA和RNA癌症生物标志物,包括HRD,以评估基因组不稳定性评分(GIS)。†
TruSight Oncology 500 High-Throughput(大样本量方案)
使用与TruSight Oncology 500相同的panel内容和组织起始量类型时,一次最多可批量处理192个样本。
通过液体活检分析血浆中的循环肿瘤DNA(ctDNA),DNA panel内容与TruSight Oncology 500相似。
| Automation capability | Liquid Handling Robots |
|---|---|
| Cancer type | Pan-Cancer, Solid Tumor |
| Method | Target Enrichment, Targeted RNA Sequencing, Targeted DNA Sequencing |
| Specialized sample types | FFPE Tissue |
| Variant class | Gene fusions, Transcript variants, Single nucleotide variants (SNVs), Insertions-deletions (indels), Copy number variants (CNVs), Loss of heterozygosity (LOH), Somatic variants, Novel transcripts, Single nucleotide polymorphisms (SNPs), Structural variants |
| 内容说明 |
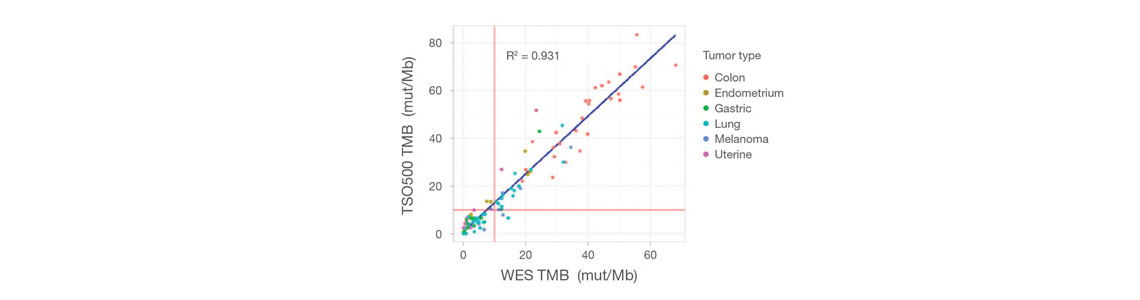
对来自523个基因的DNA和来自55个基因的RNA进行靶向测序,总panel大小为1.94 Mb。包括MSI和TMB测量。 可选的TruSight Oncology 500 HRD试剂盒(日本不可用)内容包括约25K SNP的覆盖度,可通过Myriad Genetics支持的全面基因组不稳定性评分(LOH+TAI+LST)评估同源重组缺陷。 |
| 分析时间 |
从样本到结果需要4-5天 |
| 多重分析 | 多达8重 |
| 手动操作时间 |
自动化工作流程约2.5小时。 手动工作流程约10.5小时。 |
| 技术 | Illumina测序 |
| 核酸类型 | DNA, RNA |
| 物种 | 人 |
| 系统 | NextSeq 550 Desktop Sequencer, NextSeq 550Dx in Research Mode, NextSeq 500 Desktop Sequencer |
| 自动化详情 | 探索可用的自动化方法 |
| 说明 |
该检测能够对FFPE组织进行全面的基因组分析,并在NextSeq 550系统或NextSeq 550Dx仪器(研究模式)上运行,一次最多可批量处理8个样本。 |
| 起始量 |
40 ng DNA和/或40 ng RNA |
要运行TSO 500,您需要:
要使用DRAGEN变异检出流程进行分析,您需要:
要生成最终报告,您需要:
TruSight Oncology 500能够对FFPE肿瘤样本进行全面的基因组分析,使用更少的样本评估各种生物标志物,与单基因或小型panel检测相比,结果更多。
TruSight Oncology 500检测
| 仪器 | Recommended number of samples | Read length |
|---|---|---|
| NextSeq 500 System | 8 samples per run (high output flow cell), 100M paired-end reads, 3,500× coverage |
2 × 101 bp |
| NextSeq 550Dx in Research Mode | 8 samples per run (high output flow cell), 100M paired-end reads, 3,500× coverage |
2 × 101 bp |
我们的临床癌症研究解决方案能提供准确的基因组信息,让实验室能够在单次检测中分析多个基因。
新一代测序使免疫肿瘤学研究人员能够深入了解与免疫疗法响应有关的因素和肿瘤的免疫逃避机制。
与单基因和基于阵列的方法相比,基于 NGS 的癌症测序方法可在更短的时间内提供更多信息。
| TruSight Oncology 500 | TruSight Oncology 500 High-Throughput(大样本量方案) | TruSight Oncology 500 ctDNA v2 | |
|---|---|---|---|
| Cancer type | Solid Tumor | Pan-Cancer, Solid Tumor | Pan-Cancer, Solid Tumor |
| Variant class | Gene fusions, Transcript variants, Single nucleotide variants (SNVs), Insertions-deletions (indels), Copy number variants (CNVs), Loss of heterozygosity (LOH), Somatic variants, Novel transcripts, Single nucleotide polymorphisms (SNPs), Structural variants | Gene fusions, Transcript variants, Single nucleotide variants (SNVs), Insertions-deletions (indels), Copy number variants (CNVs), Novel transcripts, Structural variants, Somatic variants | Single nucleotide variants (SNVs), Insertions-deletions (indels), Copy number variants (CNVs) |
| 内容说明 |
对来自523个基因的DNA和来自55个基因的RNA进行靶向测序,总panel大小为1.94 Mb。包括MSI和TMB测量。 可选的TruSight Oncology 500 HRD试剂盒(日本不可用)内容包括约25K SNP的覆盖度,可通过Myriad Genetics支持的全面基因组不稳定性评分(LOH+TAI+LST)评估同源重组缺陷。 |
靶向测序523个目标基因的DNA和55个基因的RNA,panel大小总计为1.94 Mb。包括MSI和TMB测量。 |
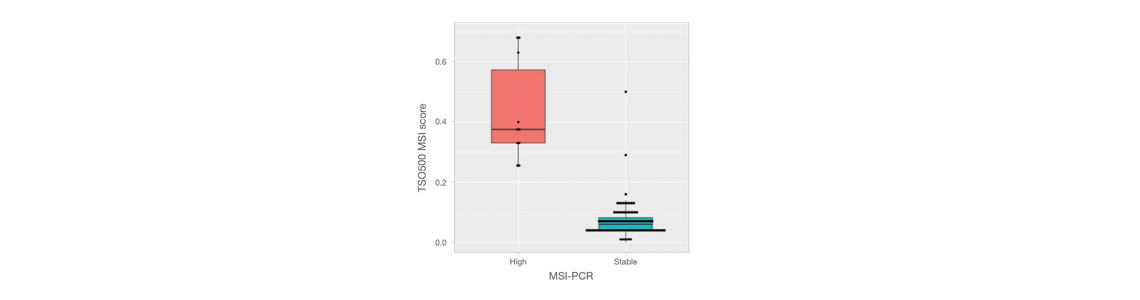
靶向选择523个基因(完整编码序列),panel总共1.94 Mb。 • 免疫肿瘤学生物标志物覆盖度:TMB和MSI • 指南覆盖范围:广泛覆盖多种实体瘤的重要指南 • 临床试验覆盖范围:600多项临床试验(基于Pierian临床知识库,截至2023年2月) |
| 分析时间 |
从样本到结果需要4-5天 |
从样本到结果需要4-5天 |
从纯化核酸到变异报告,<4天 |
| 多重分析 | 多达8重 |
多达16重(SP流动槽)、32重(S1流动槽)、72重(S2流动槽)和192重(S4流动槽) |
在S2上可进行8重分析,在S4上可进行24重分析(使用Xp-4 Lane工作流程,最多16个标签) |
| 手动操作时间 |
自动化工作流程约2.5小时。 手动工作流程约10.5小时。 |
自动化工作流程约2.5小时。 手动工作流程约10.5小时。 |
文库制备和富集约2.5小时 |
| 物种 | 人 | 人 | 人 |
| 自动化详情 | 探索可用的自动化方法 | 探索可用的自动化方法 | |
| 说明 |
该检测能够对FFPE组织进行全面的基因组分析,并在NextSeq 550系统或NextSeq 550Dx仪器(研究模式)上运行,一次最多可批量处理8个样本。 |
一种高通量的综合NGS检测方法,利用NovaSeq 6000系统或NovaSeq 6000Dx仪器(研究模式),通过简化的工作流程鉴定指南和>1K临床试验中的关键生物标志物。覆盖免疫肿瘤学生物标志物MSI和TMB。 |
通过液体活检样本(血浆中的ctDNA)的全景基因组图谱分析,提供了一种非侵入性方法,用于癌症研究应用的实体瘤图谱分析。这种液体活检方法利用微创样本采集方法来补充基于组织的CGP,提供了有关肿瘤内和肿瘤间异质性的见解。 |
| 起始量 |
40 ng DNA和/或40 ng RNA |
40 ng DNA和/或40–80 ng RNA |
20 ng cfDNA(4 mL血浆) |




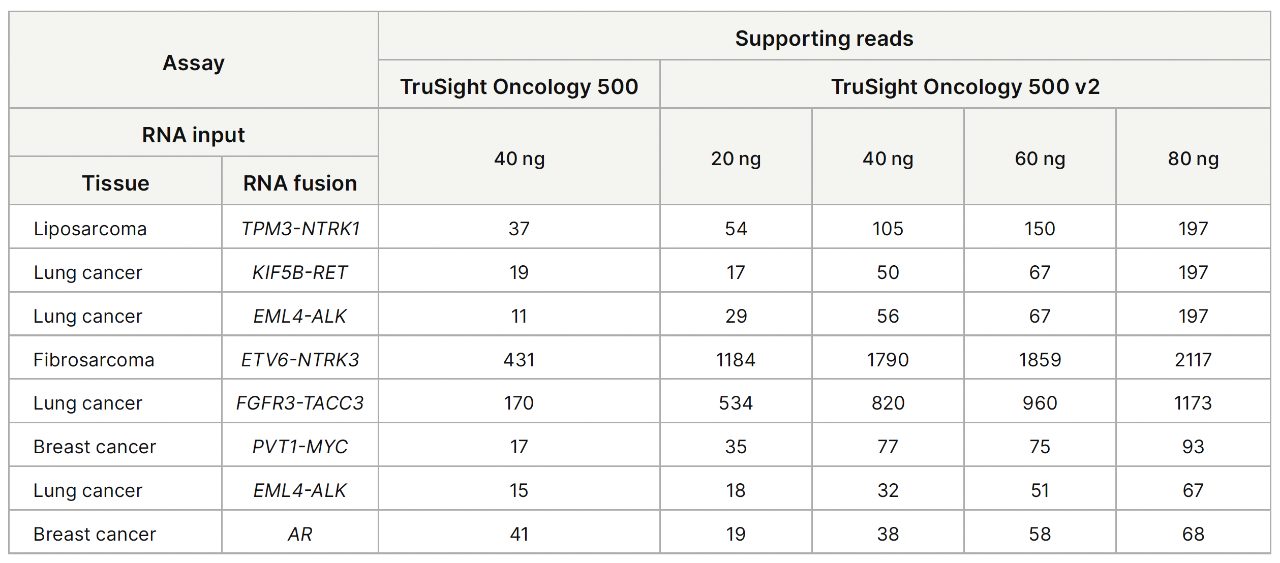

| 泛癌种:BRAF、NTRK1、NTRK2、NTRK3、RET、MSI、TMB | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 有意义的生物标志物基因* | 具有潜在意义的生物标志物基因† | ||||||||||
 乳腺癌 |
BRCA1 | BRCA2 | ERBB2 | ESR1 | PALB2 | PIK3CA | 180 | ||||
 结直肠癌 |
ERBB2 | KRAS | NRAS | 166 | |||||||
 骨癌 |
EGFR | ERG | ETV1 | ETV4 | EWSR1 | FEV | FLI1 | FUS | H3F3A | HEY1 | 140 |
| IDH1 | MDM2 | NCOA2 | SMARCB1 | ||||||||
 肺癌 |
ALK | EGFR | ERBB2 | KRAS | MET | NUTM1 | ROS1 | 223 | |||
 黑色素瘤 |
KIT | NRAS | ROS1 | 172 | |||||||
 卵巢癌 |
BRCA1 | BRCA2 | FOXL2 | 149 | |||||||
 CNS |
APC | ATRX | CDKN2A | CDKN2B | EGFR | H3F3A | HIST1H3B | HIST1H3C | IDH1 | IDH2 | 140 |
| MYCN | PTCH1 | RELA | TERT | TP53 | |||||||
 前列腺癌 |
AR | ATM | BARD1 | BRCA1 | BRCA2 | BRIP1 | CDK12 | CHEK1 | CHEK2 | FANCL | 151 |
| FGFR2 | FGFR3 | PALB2 | RAD51B | RAD51C | RAD51D | RAD54L | |||||
 甲状腺癌 |
HRAS | KRAS | NRAS | RET | TERT | 165 | |||||
 子宫和宫颈 |
BRCA2 | EPC1 | ERBB2 | ESR1 | FOXO1 | GREB1 | JAZF1 | NCOA2 | NCOA3 | NUTM2A | 138 |
| NUTM2B | PAX3 | PAX7 | PHF1 | POLE | SMARCA4 | SUZ12 | TP53 | YWHAE | |||
 其他实体瘤 |
ALK | APC | ARID1A | ASPSCR1 | ATF1 | ATIC | BAP1 | BCOR | BRCA1 | BRCA2 | 152 |
| CAMTA1 | CARS | CCNB2 | CDK4 | CDKN2A | CIC | CITED2 | CLTC | COL1A1 | COL6A3 | ||
| CREB1 | CREB3L1 | CREB3L2 | CSF1 | CTNNB1 | DDIT3 | DDX3X | DNAJB1 | DUX4 | EED | ||
| EGFR | ERBB2 | ERG | ETV1 | ETV4 | ETV6 | EWSR1 | FEV | FGFR2 | FGFR3 | ||
| FLI1 | FOXL2 | FOXO1 | FOXO4 | FUS | GLI1 | HEY1 | HGF | HMGA2 | IDH1 | ||
| KRAS | LEUTX | MAML3 | MDM2 | MYB | MYOD1 | NAB2 | NCOA2 | NF1 | NFATC2 | ||
| NFIB | NR4A3 | NRAS | NUTMI | NUTM2A | NUTM2B | PALB2 | PATZ1 | PAX3 | PAX7 | ||
| PDGFB | PDGFRA | PRKACA | PRKD1 | RANBP2 | ROS1 | SDHA | SDHB | SDHC | SDHD | ||
| SMARCB1 | SS18 | SSX1 | SSX2 | SSX4 | STAT6 | SUZ12 | TAF15 | TCF12 | TERT | ||
| TFE3 | TFEB | TFG | TP53 | TPM3 | TPM4 | TRAF7 | TSPAN31 | VGLL2 | WT1 | ||
| WWTR1 | YAP1 | YWHAE | ZC3H7B | ||||||||
表中列出的基因和生物标志物只是panel所含基因的一部分。有关完整的基因列表,请参见TruSight Oncology 500数据表。
参考文献:
1. 根据存档数据计算。Illumina公司 2023年。
TruSight Oncology 500 HRD Kit (24 samples)
20076480
TruSight Oncology 500 HRD Kit(包括HRD富集试剂。 不包括文库制备或测序核心试剂。 需要HRD分析许可证并单独出售。) 暂未在日本推出。
TruSight Oncology 500 DNA Kit (48 samples)
20028213
包含用于制备和富集多达48个DNA样本和16个标签的试剂。单独购买NextSeq 500/550测序试剂。
List Price:
Discounts:
TruSight Oncology 500 DNA Kit, For Use with NextSeq (48 samples)
20028214
包含用于制备和富集多达48个DNA样本、16个标签的试剂,以及NextSeq 500/550测序试剂。
List Price:
Discounts:
TruSight Oncology 500 DNA/RNA Bundle, (16 indexes, 24 samples)
20028215
包含用于制备和富集多达24个DNA和RNA样本以及16个标签的试剂。单独购买NextSeq 500/550测序试剂。
List Price:
Discounts:
TruSight Oncology 500 DNA/RNA Bundle, for use with NextSeq (16 indexes, 24 samples)
20028216
包括用于制备和富集多达24个DNA和RNA样本的试剂以及16个标签和NextSeq 500/550测序试剂。
List Price:
Discounts:
TruSight Oncology 500 DNA Kit plus Velsera interpretation report (16 indexes, 48 Samples)
20032624
包括使用Pierian Clinical Genomics Workspace制备和富集多达48个DNA样本、16个标签和数据解读报告的试剂。单独购买NextSeq 500/550测序试剂。
List Price:
Discounts:
TruSight Oncology 500 DNA Kit for Use with NextSeq plus Velsera interpretation report (16 indexes, 48 Samples)
20032625
包括用于制备和富集多达48个DNA样本和16个标签的试剂、使用Pierian Clinical Genomics Workspace的数据解读报告以及NextSeq 500/550测序试剂。
List Price:
Discounts:
TruSight Oncology 500 DNA/RNA Bundle plus Velsera interpretation report (16 indexes, 24 Samples)
20032626
包括使用Pierian Clinical Genomics Workspace制备和富集多达24个DNA和RNA样本的试剂,以及16个标签和数据解读报告。单独购买NextSeq 500/550测序试剂。
List Price:
Discounts:
TruSight Oncology 500 DNA/RNA Kit for Use with NextSeq plus Velsera interpretation report (16 indexes, 24 Samples)
20032627
包括用于制备和富集多达24个DNA和RNA样本以及16个标签的试剂、使用Pierian Clinical Genomics Workspace的数据解读报告以及NextSeq 500/550测序试剂。
List Price:
Discounts:
TruSight Oncology 500 DNA Automation Kit (16 indexes, 64 Samples)
20045504
包含用于制备和富集多达64个DNA样本和16个标签的试剂。单独购买NextSeq 500/550测序试剂。用于自动化。
List Price:
Discounts:
TruSight Oncology 500 DNA Automation Kit, For Use with NextSeq (16 indexes, 64 Samples)
20045505
包含用于制备和富集多达64个DNA样本和16个标签的试剂以及NextSeq 500/550测序试剂。用于自动化。
List Price:
Discounts:
TruSight Oncology 500 DNA Automation Kit plus Pierian interpretation report (16 indexes, 64 samples)
20045506
包括使用Pierian Clinical Genomics Workspace制备和富集多达64个DNA样本的试剂、16个标签和数据解读报告。单独购买NextSeq 500/550测序试剂。用于自动化。
List Price:
Discounts:
TruSight Oncology 500 DNA Automation Kit plus Pierian interpretation report, for Use with NextSeq (16 indexes, 64 samples)
20045507
包括用于制备和富集多达64个DNA样本和16个标签的试剂、使用Pierian Clinical Genomics Workspace的数据解读报告以及NextSeq 500/550测序试剂。用于自动化。
List Price:
Discounts:
TruSight Oncology 500 DNA/RNA Automation Kit (16 indexes, 32 Samples)
20045508
包含用于制备和富集多达32个DNA和RNA样本以及16个标签的试剂。单独购买NextSeq 500/550测序试剂。用于自动化。
List Price:
Discounts:
TruSight Oncology 500 DNA/RNA Automation Kit plus Pierian interpretation report (16 indexes, 32 Samples)
20045509
包括使用Pierian Clinical Genomics Workspace制备和富集多达32个DNA和RNA样本的试剂,以及16个标签和数据解读报告。单独购买NextSeq 500/550测序试剂。用于自动化。
List Price:
Discounts:
TruSight Oncology 500 DNA/RNA Automation Kit, For Use with NextSeq (16 indexes, 32 Samples)
20045990
包括用于制备和富集多达32个DNA和RNA样本的试剂以及16个标签和NextSeq 500/550测序试剂。用于自动化。
List Price:
Discounts:
TruSight Oncology 500 DNA/RNA Automation Kit plus Pierian interpretation report, For Use with NextSeq (16 indexes, 32 Samples)
20045991
包括用于制备和富集多达32个DNA和RNA样本以及16个标签的试剂、使用Pierian Clinical Genomics Workspace的数据解读报告以及NextSeq 500/550测序试剂。用于自动化。
List Price:
Discounts:
TruSight Oncology 500 Training (DNA, optional HRD) - Customer Site
20031667
客户现场为期三天的实践指导,帮助用户熟悉TruSight Oncology 500工作流程的基本步骤。本课程为最多四名学员提供样本和文库制备、富集、测序和数据分析的实践培训。
List Price:
Discounts:
TruSight Oncology 500 Training (DNA and RNA, optional HRD) - Customer Site
20031668
在客户现场提供3.5天的手动操作指导,帮助用户熟悉TruSight Oncology 500工作流程的基本步骤。本课程为最多四名学员提供样本和文库制备、富集、测序和数据分析的实践培训。
List Price:
Discounts:
Illumina Connected Insights‒Training and Onboarding
20092376
Informatics Professional Services
20071787
Illumina DRAGEN Server v3
20040619
包括第一年的Avance Exchange支持。需要购买年度DRAGEN许可证。
Illumina DRAGEN Server v4
20051343
包括第一年的Avance Exchange支持。需要购买年度DRAGEN许可证。
List Price:
Discounts:
DRAGEN TruSight Oncology 500 HRD Analysis Software, On-Premise
20073738
包括TSO 500 HRD的本地分析软件许可证。
ICA Basic Annual Subscription
20044874
Illumina Connected Analytics(ICA)基础版年度订阅。该产品为TSO500客户免费提供1年ICA,包括测序仪器连接、数据管理功能、使用预先打包的工具。
ICA Professional Annual Subscription
20044876
Illumina Connected Analytics(ICA)专业版年度订阅。该产品包括1年的ICA访问权,包括测序仪器连接、数据管理功能、预包装工具的访问权,以及创建由工具、流程、数据仓库和笔记本组成的定制工作流程的能力。
ICA Enterprise Annual Subscription
20038994
Illumina Connected Analytics(ICA)企业版年度订阅。该产品包括1年的ICA企业版访问权,包括测序仪器连接、数据管理功能、定制和预包装分析工具,以及用于数据仓储和挖掘的基础模块。ICA Enterprise还包括可选的HIPPAA BAA(仅限美国)、单点登录(SSO)和服务水平协议(SLA)。
ICA Enterprise Service and Compliance Add-on (applies to Basic only)
20066830
Illumina Connected Analytics(ICA)企业服务和合规性插件。本产品适用于ICA基础年度订阅1年,包括HIPPAA BAA(仅限美国)、单点登录(SSO)和服务水平协议(SLA)。
Illumina Analytics - 1 iCredit
20042038
iCredit用于在BaseSpace Sequence Hub或Illumina Connected Analytics上进行的数据存储和分析。
List Price:
Discounts:
Illumina Analytics Starter Pack - 1,000 iCredits
20042039
iCredit用于在BaseSpace Sequence Hub或Illumina Connected Analytics上进行的数据存储和分析。
List Price:
Discounts:
Illumina Analytics - 5,000 iCredits
20042040
iCredit用于在BaseSpace Sequence Hub或Illumina Connected Analytics上进行的数据存储和分析。
List Price:
Discounts:
Illumina Analytics - 50,000 iCredits
20042041
iCredit用于在BaseSpace Sequence Hub或Illumina Connected Analytics上进行的数据存储和分析。
List Price:
Discounts:
Illumina Analytics - 100,000 iCredits
20042042
iCredit用于在BaseSpace Sequence Hub或Illumina Connected Analytics上进行的数据存储和分析。
List Price:
Discounts:
Illumina Connected Insights-Annual Subscription
20090137
Illumina Connected Insights‒Oncology Genome Equivalent Sample-VCF
20090138
显示 /
产品
数量
Unit Price
产品
货号
数量
单价
两种检测之间的主要区别在于规模。TruSight Oncology 500在NextSeq 550或NextSeq 550Dx仪器(研究模式)上运行,一次最多可批量处理8个样本。TruSight Oncology 500 HT在NovaSeq 6000系统或NovaSeq 6000Dx仪器(研究模式)上运行,一次可批量处理16–192个样本,具有可扩展性,可提高样本通量。有关其他差异,请参见本页的比较表。
分析经过优化,最小读长为2×101 bp。低至75 bp的读长可能有效,但这尚未得到充分验证。
是的,您可以使用供应商开发的方法自动化TruSight Oncology 500工作流程。
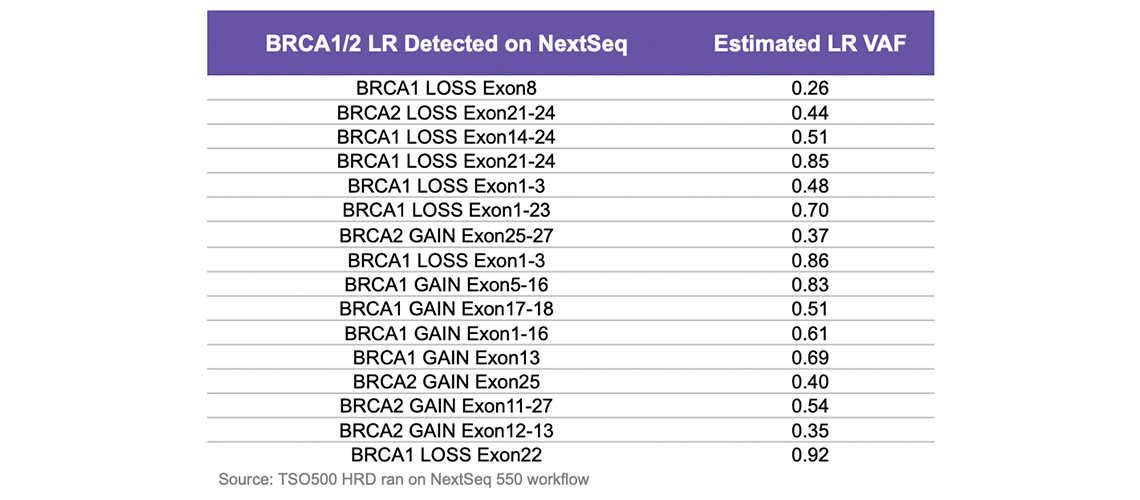
Illumina DRAGEN二级分析平台以非DRAGEN选项两倍的速度提供更高的变异检出准确性1。更高的准确性为样本QC、复杂变异、小变异、融合和剪接变异以及BRCA1/BRCA2的外显子水平CNV检出提供了更好的性能。DRAGEN流程还与TruSight Oncology 500 HRD检测兼容,可用于HRD鉴定。
是的,您可以使用自己的软件进行分析。不过,在此情况下,因美纳将无法直接提供技术支持。
填写表格,接收有关首次购买TruSight Oncology 500计划的信息。
Your email address is never shared with third parties.